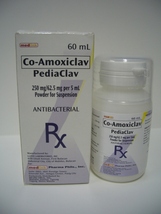
PEDIACLAV
(Co-Amoxiclav)
FORMULATION:
Each 5 mL (1 teaspoonful) of reconstituted suspension contains:
Amoxicillin (as trihydrate) …………….………………….……………. 250 mg
Clavulanic Acid (as clavulanate potassium) .………………….. 62.5 mg
MECHANISM OF ACTION:
Amoxicillin acts through inhibition of biosynthesis of the bacterial cell wall mucopeptide. It is bactericidal against susceptible organisms during the stage of active multiplication. Amoxicillin is active against many Gram-positive and Gram-negative pathogens. However, it is susceptible to degradation by beta-lactamases and therefore its spectrum does not include organisms which produce these enzymes. Clavulanic acid is a beta lactam structurally related to the penicillins, found in micro-organisms resistant to penicillins.
The formulation of amoxicillin with clavulanic acid protects amoxicillin from degradation by beta-lactamase enzymes and effectively extends the antibiotic spectrum of amoxicillin to include many bacteria normally resistant to amoxicillin and other beta-lactam antibiotics.
PHARMACOKINETICS:
Amoxicillin and clavulanate potassium are both well absorbed after oral administration and are stable in the presence of gastric acid. Food does not affect the absorption and this combination product may be given without regard to meals. The oral bioavailability of amoxicillin and clavulanic acid is approximately 90% and 75 % respectively.
Clavulanic acid has about the same plasma elimination half-life (1 hour) as that of amoxicillin (1.3 hrs.)
Amoxicillin and clavulanic acid are widely distributed to most tissues and body fluids including peritoneal fluid, blister fluid, urine, pleural fluid, middle ear fluid, intestinal mucosa, bone, gallbladder, lungs, female reproductive tissues and bile. The penetration into CSF through non-inflammed meninges and into purulent bronchial secretions is low. Amoxicillin and clavulanic acid readily cross the placenta and are distributed into breast milk in low concentrations. Amoxicillin is bound to serum proteins to an extent of 17-20% while clavulanic acid is 20-30 % bound to serum proteins. Approximately 10 % of the dose of amoxicillin and less than 50 % of dose of clavulanic acid are metabolized.
Amoxicillin and clavulanic acid are eliminated primarily unchanged through the renal route (glomelular filtration and tubular secretion). Approximately 50-78 % of amoxicillin and 25-40% of clavulanic acid are excreted unchanged in urine within the first 6 hours after administration.
INDICATIONS:
Co-amoxiclav is indicated for the treatment of following infections caused by susceptible pathogens: lower respiratory tract infections, acute otitis media, sinusitis, urinary tract infections, skin and soft tissue infections.
Co-amoxiclav is also indicated for bacterial infections likely to be caused by amoxicillin-resistant beta-lactamase producing strains and that treatment should not usually exceed 14 days.
It is also considered for the following indications:
- recurrent tonsillitis
- acute exacerbations of chronic bronchitis, bronchopneumonia
- urinary tract infection especially recurrent & complicated but not prostatitis
- septic abortion, pelvic or puerperal sepsis & intra-abdominal sepsis
- cellulitis, animal bites and severe dental abscess with spreading cellulitis
DOSAGE AND ADMINISTRATION:
The usual adult oral dosage of Amoxicillin and Clavulanate potassium is one tablet containing 250 mg of Amoxicillin and 125 mg of Clavulanic acid every 8 hours. For more severe infections and infections of respiratory tract, the usual oral dosage is one tablet containing 500 mg of Amoxicillin and 125 mg of Clavulanic acid every 8 hours. Or as prescribed by the physician.
Suspension
7-10 years: 10 mL (2 teaspoonfuls) of 125 mg/31.25 mg suspension every 8 hours.
Or
5 mL (1 teaspoonful) of 250 mg/62.5 mg suspension every 8 hours
2-6 years: 5 mL (1 teaspoonful) of 125 mg/31.25 mg suspension every 8 hours.
or under 20 kg bodyweight dose of 20-40 mg per kg daily in divided doses every 8 hours.
9 months-1 year: 2.5 mL (½ teaspoonfuls) of 125 mg/31.25 mg suspension every 8 hours.
Or as prescribed by the physician.
PRECAUTIONS:
Changes in liver function tests have been observed in some patients receiving Co-amoxiclav. Therefore dosage should be reduced for patients with severe renal impairment. The clinical significance of these changes is uncertain but Co-amoxiclav should be used with caution in patients with evidence of hepatic dysfunction. Cholestatic jaundice, which may severe, but is usually reversible, has been reported rarely. Signs and symptoms may not become apparent for up to six weeks after treatment ceased. Erythematous rashes have been associated with granular fever in patients receiving amoxicillin. Co-amoxiclav should be avoided if glandular fever is suspected. Prolonged use may also occasionally result in overgrowth of non-susceptible organisms.
ADVERSE EFFECTS:
Nausea, vomiting, diarrhea, rashes, rarely antibiotic associated colitis.
CONTRAINDICATIONS:
A history of allergic reactions to beta-lactam antibiotics penicillin hypersensitivity, history of co-amoxiclav associated or penicillin associated jaundice or hepatic dysfunction.
DIRECTION FOR RECONSTITUTION:
250 mg/62.5 mg per 5 mL Powder for suspension:
To make 60 mL reconstituted suspension, mix thoroughly the contents with 50 mL cooled water and shake well until the powder is evenly suspended. After reconstitution, suspension is stable for 7 days under refrigeration (2-8°C).
CAUTION:
Foods, Drugs, Devices and Cosmetics Act prohibits dispensing without prescription.
STORAGE CONDITION:
Store at temperatures not exceeding 25ºC
Shake well before using
AVAILABILITY:
250 mg/62.5 mg per 5 mL Powder for Suspension
Plastic Bottle -Bottle of 60 mL
Manufactured By:
LLOYD LABORATORIES, INC.
#10 Lloyd Avenue, First Bulacan Industrial City,
City of Malolos, Bulacan
For:
MEDLINK PHARMA PHILS., INC
Suite 2002, 20/F, Prestige Tower,
Emerald Ave., Ortigas Center, Pasig City
Exclusively Distributed by:
METRO DRUG, INC.
Mañalac Avenue, Tanyag, Taguig, Metro Manila
<< Back to All Products