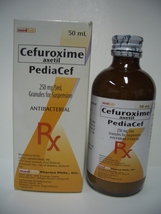
PEDIACEF
(Cefuroxime axetil)
FORMULATION:
Each 5 mL (1 teaspoonful) reconstituted suspension contains:
Cefuroxime (as axetil) ……………………………...250 mg
ANTIMICROBIAL ACTION:
Cefuroxime is bactericidal and has a similar spectrum of antimicrobial action and pattern of resistance to those of cefamandole. It is more resistant to hydrolysis by beta-lactamases than cefamandole, and therefore may be more active against beta-lactamase producing strains, Haemophilus influenza and Neisseria gonorrhea. However, treatment failures have occurred in patients with H. influenzae meningitis given cefuroxime and might be associated with a relatively high minimum bactericidal concentration when compared with the minimum inhibitory concentration or with a significant inoculums effect. Reduced affinity of penicillin-binding proteins for cefuroxime has also been reported to be responsible for resistance in a beta-lactamase-negative strain of H.influenzae.
PHARMACOKINETICS:
Cefuroxime axetil is absorbed from the gastrointestinal tract and is rapidly hydrolyzed in the intestinal mucosa and blood to cefuroxime; absorption is enhanced in the presence of food. Peak plasma concentrations are reported about 2 to 3 hours after an oral dose. The sodium salt is given by intramuscular or intravenous injection. Peak plasma concentrations of about 27 ug per mL have been achieved 45 minutes after an intramuscular dose of 750 mg with measurable amounts present 8 hours after a dose. Up to 50% of cefuroxime in the circulations is bound to plasma proteins. The plasma half-life is about 70 minutes and is prolonged in patients with renal impairment and in neonates.
PHARMACOLOGIC:
There are 2 groups within the 2nd generation agents that differ in their: 1) spectrum of activity and 2) adverse reaction profile. These groups are the "true" second generation cephalosporin (cefamandole, cefuroxime) and the cephamycins (cefoxitin, cefotetan, cefmetazole).
SPECTRUM OF ACTIVITY. Gram-positive aerobic cocci: In general, true 2nd generation agents are comparable to 1st generation agents against non-enterococcal streptococci; are less active in-vitro, but still have adequate activity against MSSA. Compared to the 1st generation agents, the cephamycins are less active against gram-positive cocci. Both groups of cephalosporins are inactive against methicillin-resistant Staphylococci and Enterococci.
Gram-negative aerobes. The "true" cephalosporins are more active than 1sts for Hemophilus influenzae, Moraxella catarrhalis, Neisseria meningitides, and some Enterobacteriaceae. The cephamycins in some instances (e.g.: cefotetan) have improved activity against Enterobacteriaceae.
Anaerobes: Cephamycins are active against most anaerobes found in the mouth as well as colon (e.g.: Bacteroides species, including Bacteroides fragilis).
INDICATIONS:
Used in the treatment of susceptible infections. These include bone and joint infections, bronchitis, gonorrhea, meningitis (although treatment failures have been reported in H. influenzae meningitis), otitis media, peritonitis, pharyngitis, sinusitis, skin infections (including soft-tissue infections), and urinary tract infections. Applicable for parenteral dosage form.
PRECAUTIONS:
Cefuroxime should not be given to patients who are hypersensitive to it or to other cephalosporins. About 10% of penicillin-sensitive patients may also be allergic to cephalosporins although the true incidence is uncertain; great care should be taken if cefalotin is to be given to such patients. Care is also necessary in patients with a history of allergy.
ADVERSE EFFECTS:
The most common are hypersensitivity reactions, including skin rashes, urticaria, eosinophilia, fever, reactions resembling serum sickness, and anaphylaxis.
Gastrointestinal disturbances, including diarrhea, nausea, and vomiting, have occurred in some patients receiving cefuroxime axetil. There have been rare reports of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Mild to moderate hearing loss has been reported in some children given cefuroxime for the treatment of meningitis.
DOSAGE AND ADMINISTRATION:
Children over 2 years of age with otitis media may be given 250 mg twice daily or 15 mg per kg twice daily to a maximum of 500 mg daily or as prescribed by the physician.
Children more than 3 months of age – 125 mg twice daily or 10 mg per kg body weight twice daily to a maximum of 250 mg daily or as prescribed by the physician.
DIRECTION FOR RECONSTITUTION:
250 mg/5 mL Granules for Suspension
To make 50 mL reconstituted suspension, add approximately 25 mL of water. Shake well until the powders are evenly suspended. The suspension is stable for one week at temperatures not exceeding 30°C and two weeks under refrigeration (2-8°C).
CAUTION:
Foods, Drugs, Devices and Cosmetics Acts prohibits dispensing without prescription.
STORAGE CONDITION:
Store at temperatures not exceeding 30ºC
Shake well before use
AVAILABILITY:
Amber bottle x 120 mL (content of 50 mL)
In Raspberry Flavor
Manufactured by:
LLOYD LABORATORIES, INC.
#10 Lloyd Ave., First Bulacan Industrial City,
City of Malolos, Bulacan
For:
MEDLINK PHARMA PHILS., INC.
Suite 2002, 20/F, Prestige Tower,
Emerald Ave., Ortigas Center, Pasig City
Exclusively Distributed by:
METRO DRUG, INC.
Mañalac Avenue, Tanyag, Taguig , Metro Manila
<< Back to All Products