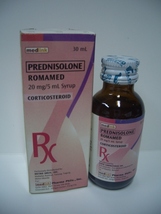
ROMAMED
(Prednisolone)
The Great-tasting, Most Affordable Liquid Prednisolone
Romamed Syrup is the great tasting reliable, and most affordable liquid prednisolone—great benefits for your long-suffering pediatric asthmatic patients. So try Romamed syrup, available in 30mL, bottles with 20mg prednisolone per 5mL.
What is the Mode of Actions of Romamed?
Romamed is prednisolone, a synthetic corticosteroid. Romamed works by acting within cells to prevent the release of certain chemicals that are important in the immune system. These chemicals are normally involved in producing-immune and allergic responses, resulting in inflammation. By decreasing the release of these chemicals in a particular area, inflammation is reduced. This can help control a wide number of disease states, characterized by excessive inflammation. They include severe allergic reactions, inflammation of the lungs in asthma and inflammation of the joints in arthritis.
FORMULATION:
Each 5 mL (1 teaspoonful) contains:
Prednisolone Phosphate (as Sodium) ..................... 20 mg
DESCRIPTION:
Prednisolone (Romamed) is a glucocorticoid given, as the free alcohol or in esterified form, orally or parenterally, in the treatment of various disorders in which corticosteroids are indicated, except adrenal deficiency states. It is also given by local injection, applied rectally and applied topically as eye drops or ear drops. Adverse effects are those of corticosteroids in general. It has relatively slight mineralocorticoid effects.
PHARMACOKINETICS:
Prednisolone (Romamed) and prednisone are both readily absorbed from the gastrointestinal tract, but whereas Prednisolone (Romamed) already exists in metabolically active form, prednisone must be converted in the liver to its active metabolite, Prednisolone. In general, this conversion is rapid so that prednisone has a preconversion biological half-life of only about 60 minutes. Hence, although prednisone has been estimated to have only about 80% the bioavailability of Prednisolone (Romamed), this difference of little consequence when seen in the light of intersubject variation in the pharmacokinetics of Prednisolone (Romamed) itself; bioavailability also depends on the dissolution rates of the tablet formulations. Nevertheless, Prednisolone (Romamed) is the more reliably absorbed of the two corticosteroids, particularly in some liver diseases where the conversion of prednisone may be diminished. Peak plasma concentrations of a Prednisolone (Romamed) are obtained 1 to 2 hours after administration by mouth, and it has a usual half-life of 2 to 4 hours. Its initial absorption, but not its overall bioavailability, is affected by food. Prednisolone (Romamed) is extensively bound to plasma proteins, although less than hydrocortisone (cortisol). Prednisolone (Romamed) is excreted in the urine as free and conjugated metabolites, together with an appreciable proportion of unchanged prednisolone. Prednisolone (Romamed) crosses the placenta and small amounts are excreted in breast milk.
Prednisolone (Romamed) has a biologically half-life lasting several hours, intermediate between those of hydrocortisone (cortisol) and the longer-acting glucocorticoids, such as dexamethasone. It is this intermediate duration of action which makes it suitable for the alternate-day administration regimens which has been found to reduce the risk of adrenocortical insufficiency, yet provide adequate corticosteroid coverage in some disorders.
ADVERSE EFFECTS:
Large doses of corticosteroids or of corticotrophin, may produce Cushingoid symptoms typical of hyperactivity of the adrenal cortex, with moon-face, sometimes hirsutism, buffalo hump, flushing, increased bruising, ecchymoses, striae and acne, sometimes leading to a fully developed Cushing’s syndrome. If administration is discontinued these symptoms are usually reversed, but sudden cessation is dangerous.
Other adverse effects include amenorrhea, hyperhidrosis, skin thinning, ocular changes including development of cataract, mental and neurological disturbances, intracranial hypertension, acute pancreatitis, and aseptic necrosis of the bone. An increase in coagulability of the blood may lead to thrombo-embolic complications. Peptic ulceration has been reported. Muscle weaknesses and wasting occur occasionally, particularly when corticosteroids are taken in large doses. The former arises from the mineralocorticoid properties of corticosteroids, the latter from their glucocorticoid properties and its most evident with triamcinolone.
DRUG INTERACTION:
Concurrent use of barbiturates, cerbamazepine, phenytoin, primidone, or rifampicin may enhance the metabolism and reduce the effects of systemic corticosteroids. Conversely oral contraceptives or ritonavir may increase plasma concentrations of corticosteroids. Use of corticosteroids with potassium-depleting diuretics, such as thiazides or frusemide (furosemide) may cause excessive potassium loss. There is also an increased risk of hypokalaemia with concurrent amphotericin B or bronchodilator therapy with xanthines or beta 2 agonists. There may be an increased incidence of gastrointestinal bleeding and ulceration when corticosteroids are given with NSAIDs. Response to anticoagulants may be altered by corticosteroids and requirements of antidiabetic drugs and antihypertensives may be increased. Corticosteroids may decrease serum concentrations of salicylates and may decrease the effect of antimuscarinies in myasthenia gravis.
PRECAUTION:
The dose of corticosteroid required to diminish corticotrophin secretion with consequent atrophy of the adrenal cortex and the time required for its occurrence vary from patient to patient. Acute adrenal insufficiency may occur during prolonged treatment or on cessation of treatment and may be precipitated by stressful situation. Growth retardation in children has been reported. High doses of corticosteroids administered during pregnancy may cause fetal or neonatal adrenal suppression
INDICATION:
Prednisolone (Romamed) is used to control the acute exacerbations of bronchial asthma, severe osteoarthritis,severe allergic reactions,autoimmure diseases, and in rheumatoid arthritis.
DOSAGE and ADMINISTRATION:
The dose recommended in children based on a kilogram body weightis 1-2mg kg body weight given once or in divided doses. Short therapy of 3 to 5 days needs no tapering of dose. or as prescribe by a physician.
CAUTION:
Foods, Drugs, Devices, and Cosmetics Act prohibits dispensing without prescription.
AVAILABILITY:
Romamed 20 mg/ 5mL Syrup Bottle of 30 mL
STORAGE CONDITIONS:
Store at temperatures not exceeding 30°C.
Manufactured by:
LLOYD LABORATORIES, INC.
10 Lloyd Avenue, First Bulacan Industrial City, City of Malolos, Bulacan
For:
MEDLINK PHARMA PHILS., INC.
Suite 2002, 20/F Prestige Tower, Emerald Avenue, Ortigas Center, Pasig City
Exclusively Distributed by:
METRO DRUG, INC.
Mañalac Avenue, Tanyag Taguig, Metro Manila
<< Back to All Products